Development of efficient deuterium enrichment method (Topic of Pioneer Laboratory)
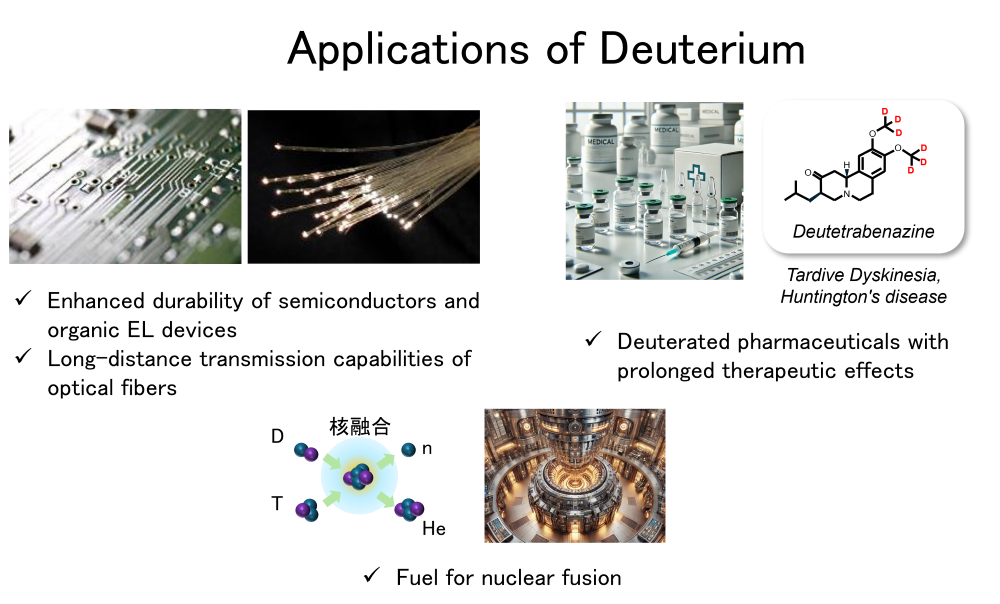
Deuterium(D), an isotope of hydrogen, exists in trace amounts in nature (approximately 0.015%), with most of it found in natural water. This rare substance plays an indispensable role in various fields, such as enhancing the durability of semiconductors and organic EL devices, improving the transmission performance of optical fibers, and enabling structural analysis of pharmaceuticals and chemical products. Furthermore, deuterium is expected to serve as a critical fuel for nuclear fusion energy in the future. Deuterium is primarily produced by concentrating it from natural water, but the process requires enormous energy costs, making it heavily reliant on imports. As a result, high import costs and supply chain risks have become significant issue. Our team is working on the development of efficient and cost-effective deuterium enrichment methods by utilizing electrochemical device techniques based on polymer electrolyte membranes (PEM), specifically PEM water electrolysis and hydrogen pumping techniques. Through these efforts, we aim to establish a sustainable deuterium supply system.
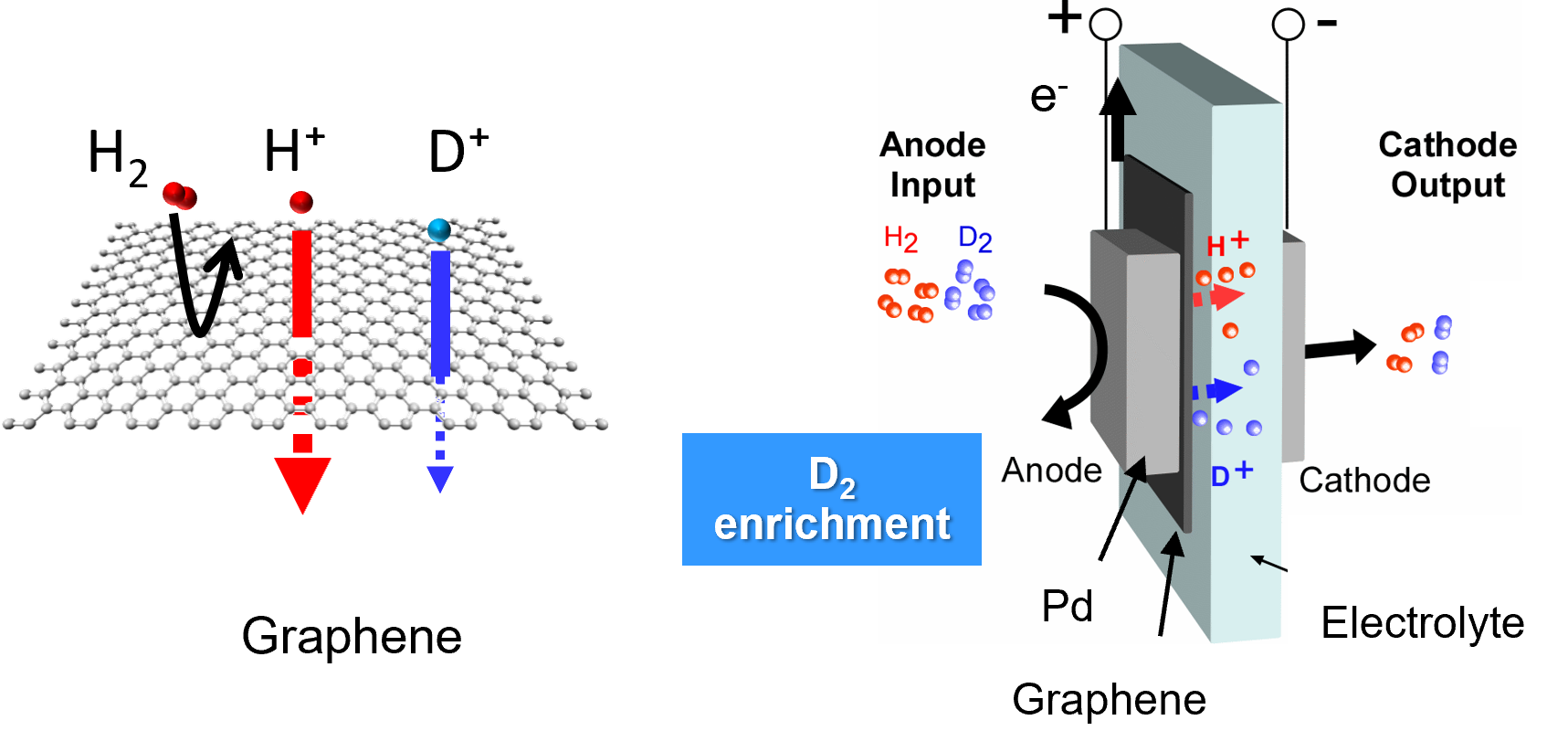
I. Enrichment of Deuterium gas using the Quantum Tunneling Effect of Graphene
It has been reported that graphene, a two-dimensional material consisting of a single atomic layer, exhibits
selective permeability for hydrogen isotope ions at room temperature. However, the mechanism underlying this
selective ion permeability remained unclear. Utilizing polymer electrolyte membrane-based electrochemical devices,
we precisely evaluated the permeation of hydrogen isotope ions through graphene. As a result, we experimentally and
theoretically demonstrated that lighter hydrogen ions permeate graphene more effectively than deuterium ions due to
the quantum tunneling effect. Based on these findings, we are working on the development of a deuterium separation
device with high separation efficiency at room temperature by leveraging the quantum tunneling effect of hydrogen
isotope ions through graphene.
Referene : ACS Nano 16, 9, 14362-14369 (2022)
II. Development of Electrode Catalysts for PEM Electrolysis with efficient Heavy Water Enrichment
When light water (H₂O) undergoes electrolysis, it decomposes into hydrogen and oxygen.
However, heavy water (D₂O) is less readily electrolyzed compared to light water. Utilizing this property,
electrolyzing a mixed aqueous solution of light water and heavy water causes the light water to decompose preferentially,
thereby concentrating the heavy water in the solution. Proton exchange membrane (PEM) electrolysis, which utilizes
a solid polymer membrane, offers advantages such as compact design, low maintenance requirements, and reduced operational
costs, making it a promising technology for heavy water enrichment. However, the current PEM electrolysis have
limited heavy water enrichment performance, necessitating further improvements. Our research focuses on developing
high-performance electrode catalysts with enhanced heavy water enrichment capabilities. In addition, we employ advanced
surface analysis techniques to elucidate the mechanisms underlying heavy water concentration. Through these efforts,
we aim to establish more efficient and high-performance technologies for heavy water enrichment.
Referene : Patent submitted
III. Development of a PEM Electrolysis System with Efficient Heavy Water Enrichment
In preparation. (Patent submitted)
IV. Modeling and Simulation of Heavy Water Enrichment via PEM Electrolysis
In preparation.
Development of Structurally Controlled Carbon Catalysts Utilizing Self-Organization Ability
Catalysts are essential to our daily lives, but with technological advancements, many catalysts rely on rare precious metals as raw materials. This raises concerns about the potential depletion of metal resources in the future. To address this issue, efforts to develop catalysts that minimize the use of precious metals are being pursued worldwide. Among these efforts, research on catalysts utilizing abundant and inexpensive elements such as carbon, nitrogen, and iron has gained significant attention. In our study, we are developing model catalysts with precisely designed structures using graphene nanoribbons with carbon frameworks. This approach allows us to finely control the catalyst structure and identify which configurations exhibit the highest catalytic activity. Through this, we aim to establish design principles for high-performance carbon-based catalysts that do not rely on precious metals and further their development.
Referene : RSC Advances 13, 14089-14096 (2023)