Measurement of the first ionization potential of lawrencium, element 103
ഀ ഀഀ
| ഀ
Measurement of the first ionization potential of lawrencium, element 103 ഀ T. K. Sato, et al., Nature, doi:10.1038/nature14342(2015). ഀ |
Our research program aims at understanding chemical, atomic, and nuclear properties of the heaviest actinides and of superheavy elements (SHEs, elements with atomic number Z ≥ 104) placed at the farthest reach of the Periodic Table of the Elements (PTE). Fig. 1 shows a photo of the research group for superheavy elements at the Advanced Science Research Center (ASRC).ഀ The highlight of our program focusing on atomic properties was the first-time measurement of the first ionization potential of lawrencium (Lr, Z = 103) [1]. Lr is the heaviest actinide element and is located at the transition into the SHEs. The result clearly reveals, in agreement with theoretical expectations, that relativistic effects play an important role in the binding of the outermost electrons with the consequence that the p1/2 valence electron of Lr is the most weekly bound valence electron among all actinides and most other chemical elements.ഀ ഀ |
|
Fig.1 Group photo of the research group for the superheavy element of the Advanced Science Research Center (ASRC) from 2014.ഀ ഀ ഀ |
[Background] ഀ |
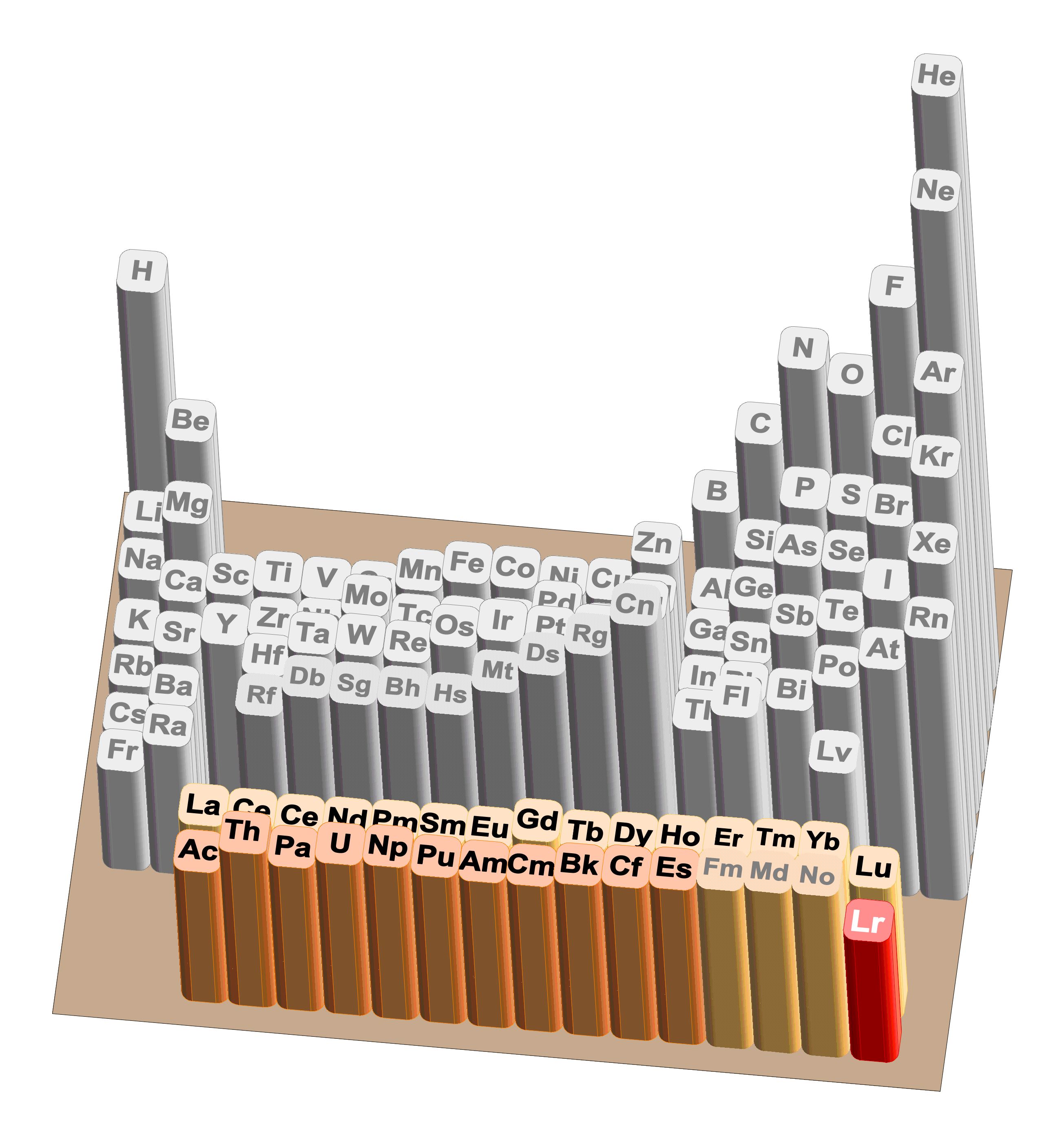
Exploring the frontiers and basic properties of matter are among the most fundamental quests in science. Here, examining the limits of existence of chemical elements and clarifying their basic properties are key topics in both chemistry and physics [2]. ഀ ഀOriginally oriented towards detailed investiga¬tions of elements in the transition region from heavy actinides to light SHEs, our research to elucidate chemical, atomic, and nuclear properties of SHEs is now focusing on opening up new frontiers with novel type and most challenging experiments at the limits of matter and technical feasibility. These experiments at the uppermost end of the PTE (Fig. 2) are mostly performed in worldwide collaborations at heavy-ion accelerators with the JAEA Tandem accelerator being a major component. ഀ ഀBecause of short half-lives and low production rates, each SHE atom produced decays before a new one is synthesized. Therefore, automated, rapid, highly efficient, and selective procedures are indispensable to determine desired properties on an "atom-at-a-time" basis [2].ഀ ഀWe focus on the valence electronic structure, which is strongly influenced by relativistic effects [2]. Experimentally we explored it by the first-ever measurements of the first ionization potential (IP1) of lawrencium; the heaviest element an ionization potential has been determined. It was also the first time Lr was successfully ionized and mass separated with a surface ionization method as part of an Isotope Separator On–Line (ISOL) [3,4]. ഀ ഀ |
|
Fig. 2 Periodic Table of the Elements.ഀ ഀ ഀ |
[The first ionization potential of 103Lr] ഀ |
Relativistic effects are strongly affecting the electronic configuration of the heaviest elements [2]. In the actinides, the relativistic expansion of the 5f orbital contributes to the actinide contraction. Together with direct relativistic effects on the 7s and 7p1/2 orbitals, this influences the binding energies of valence electrons and the energetic ordering of electronic configurations. It is, however, difficult to directly measure energetic levels of the heaviest actinides with Z > 100 by any spectroscopic method as these elements are not available in (weighable) macro amount.ഀ ഀIn comparison with theory, the measurement of the IP1 (Fig. 3), a fundamental physical property of an element, provides a test of relativistic effects which are significantly noticeable in heavy elements. The ground state electronic configuration of lawrencium is expected to be [Rn]5f147s27p1/2, which is different from that of the lanthanide homolog Lu [Xe]4f146s25d. The reason for this change is the stabilization of the 7p1/2 orbital of Lr below the 6d orbital by strong relativistic effects. Lr, therefore, is anticipated to be the first element with a 7p orbital in its electronic ground state. ഀ ഀAs the first ionization potential directly reflects the binding energy of a valence electron under the influence of relativistic effects, its experimental determination provides direct information on the energetics of the electronic orbitals of Lr, including relativistic effects, and a test for modern theories. However, this measurement cannot answer any question about the electronic configuration itself. For more information on theory aspects directly related to our Lr experiment, please, visit the web site of our collaboration partner at www.superheavies.de.ഀ ഀ |
|
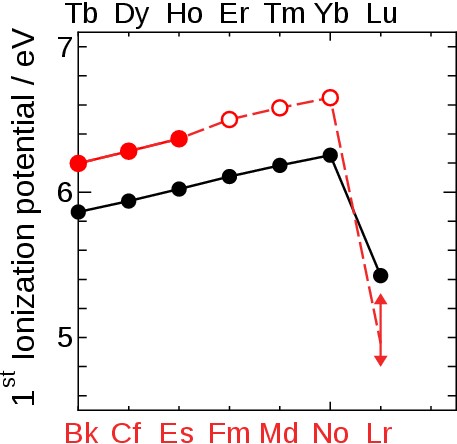
Fig. 3 First ionization potential of heavy lanthanides (black symbols) and actinides (red symbols) [3]. Closed and open symbol indicate experimental and estimated values, respectively. For Lr the range of IP1 values from theoretical calculations made over the last two decades is shown.ഀ ഀ ഀ To experimentally determine the IP1 of Lr, we applied a surface ionization process. Here, an atom is ionized via interaction with a solid surface kept at a high temperature. In this process, the ionization efficiency (Ieff) depends on a material sensitive “work function”, the surface temperature, and the IP1 of the element. We utilized the relation between IP1 and Ieff to determine the IP1 of Lr.ഀ ഀ |
|
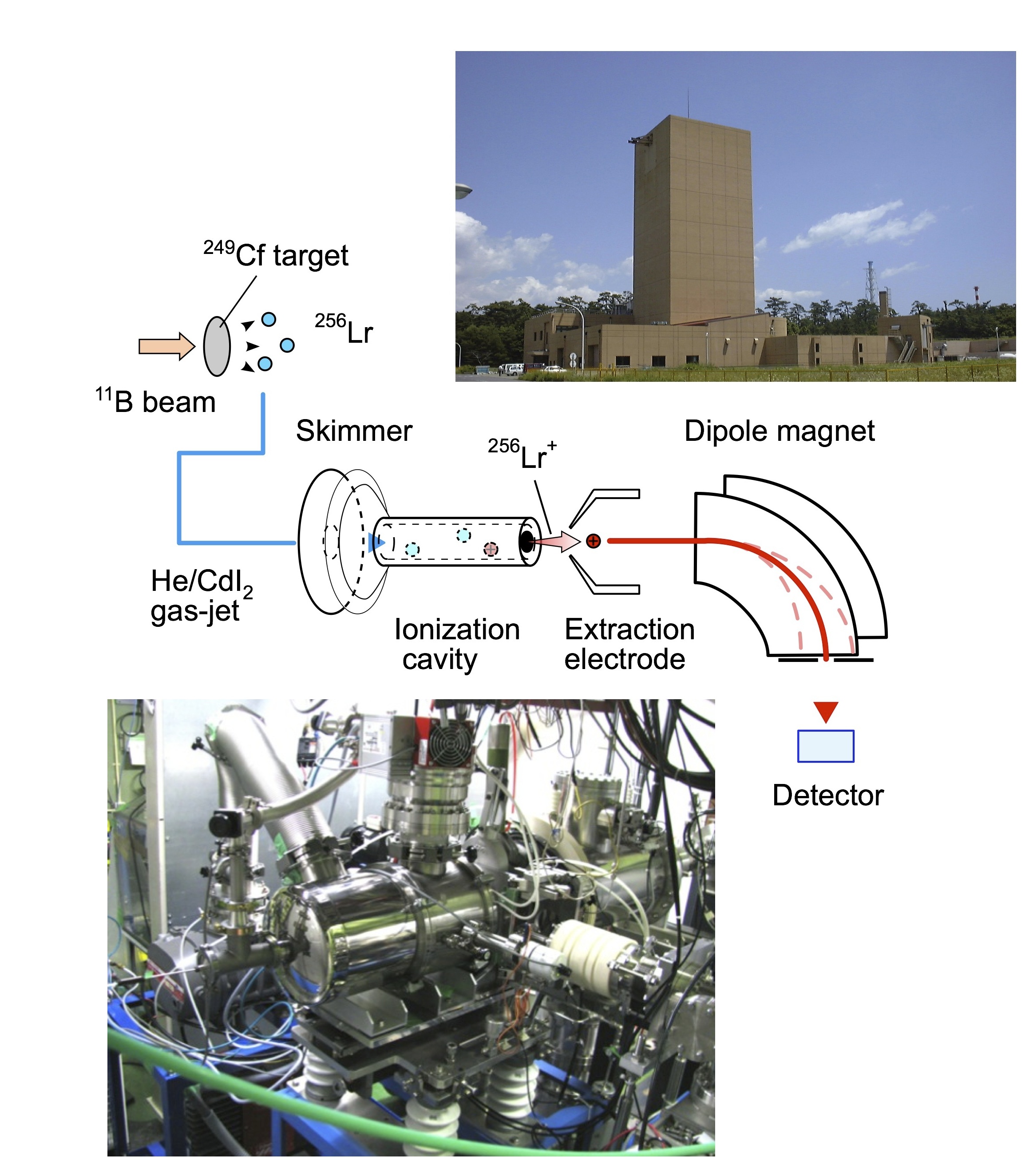
Fig. 4 Schematic view of the experimental setup for the measurement of the ionization energy of Lr together with photos for the JAEA tandem accelerator (upper right) and JAEA-ISOL (lower left). ഀ ഀ |
| ഀ
In order to perform the IP1 measurement for short-lived isotopes of Lr, we have developed and optimized a novel method based on the surface ionization process. The pivotal part of this multi-stage system is our highly efficient surface ionization ion-source [3]. The entire set-up (Fig. 4) consists of a 249Cf-target and recoil chamber, a gas-jet transport system, the above mentioned surface ion-source as part of the JAEA-ISOL (Isotope Separator On-Line), a mass-separator, and α/γ detection systems. Along this chain, Lr, recoiling from the target, was transported to the ion-source by a He/CdI2 gas-jet, was ionized to Lr+ in the ion-¬source, and was extracted and mass-separated with the mass-separator in the JAEA-ISOL [4]. The isotope 256Lr (T1/2 = 27 s), produced in the reaction of 11B on 249Cf [5], was used in these studies. ഀ ഀ The ionization experiments were performed at several temperatures of the ion-source. The number of ions collected at the end of the ISOL was determined by α-particle or γ-ray measurements. To determine the 100% value of products entering the ion-source, which is required to calculate the Ieff, in separate calibration experiments [3], all reaction products transported from the target recoil chamber were collected directly from the jet and were measured. ഀ ഀ |
|
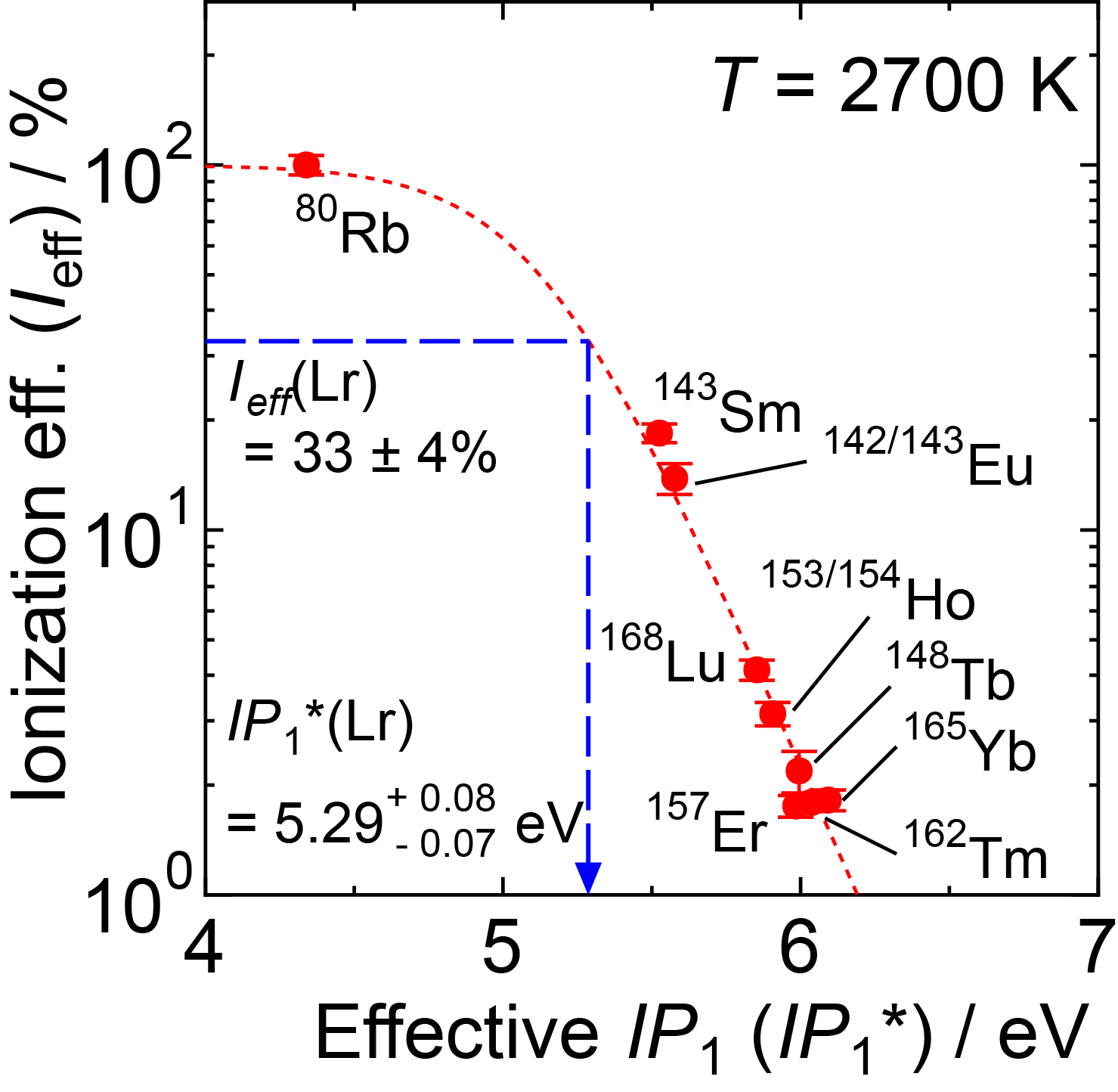
Fig. 5 The ionization efficiency (Ieff) of various short-lived isotopes as a function of the effective first ionization potential (IP1*) at 2700 K. The dotted curve is obtained by fitting the experimental data which describes a relationship between Ieff and IP1* in our present system. The position of the measured Ieff value of Lr is also shown. IP1* of Lr is calculated to be 5.29 eV. This corresponds to an IP1 value of 4.95 eV at 2700 K. ഀ ഀ |
| ഀ
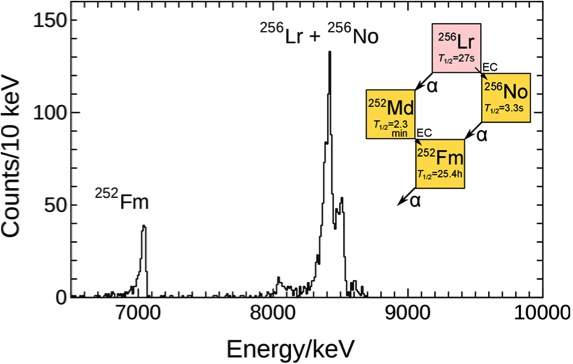
Fig. 6 shows that we successfully ionized 256Lr, mass ¬separated 256Lr+, and detected it by its radioactive decay [3]. By applying our established Ieff vs. IP1* relationship using lanthanide isotopes, the IP1* of Lr is calculated to be 5.29 eV. This corresponds to a value for the IP1 value of 4.95 eV at 2700 K. From all our experiments at 2700 K and 2800 K we obtained an average value for the ionization potential of 4.96 eV [1]. ഀ ഀ ഀ |
|
Fig. 6 Sum of alpha-spectra for the mass number 256 together with the decay scheme of 256Lr. ഀ ഀ |
| ഀ
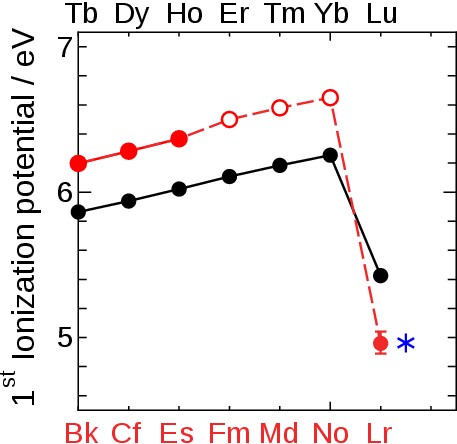
Theoretical work suggests Lr to have closed 5f14 and 7s2 shells and a very weekly-bound 7p1/2 valence electron. We determined its binding energy by measuring the first ionization potential. This successful experiment proves that this technique is applicable for investigations of atom-at-a-time man-made heavy and superheavy elements. In agreement with a state-of-the-art atomic calculation that includes relativistic effects and corrections for the Breit term and the Lamb shift [1] (see also www.superheavies.de), our measured value of 4.96 eV proofs that Lr has the most weakly-bound valence electron among all actinides; its binding is significantly weaker than in its homolog Lu and weaker than any element except group 1 elements heavier than Na; see Fig. 7. It validates the position of Lr as the last actinide element and confirms the architecture of the periodic table; see Fig. 8. ഀ |
|
Fig. 7 Ionization potential of heavy lanthanides (black symbol) and actinides (red symbol) including our present results. A closed and open symbol indicates an experimental and estimated value, respectively. The blue star * depicts the present theoretical value for Lr demonstrating the excellent agreement between theory and experiment. ഀ ഀ |
|
Fig. 8 Periodic Table of the elements with their ionization potentials. ഀ ഀ |
[Importance of the result and perspectives] ഀ |
| ഀ
Studies of atomic properties of SHEs, i.e., all elements beyond Lr, now seem to be within reach exploiting the capabilities of our most powerful JAEA-ISOL system. They can provide new insights into atomic properties of SHEs which, up to now, were not accessible. ഀ ഀIn frontier experiments we elucidated chemical and atomic properties of the heaviest elements. This significantly contributed to the advancement of the knowledge and understanding of SHEs. The influence of relativity on SHEs remains a central topic. It will be probed in future detailed experiments following the path of new and recently very successful approaches. Novel, rapid, highly efficient, and selective techniques were developed which are now at hand to push the frontiers even further in chemical studies when probing short-lived species of SHEs one atom-at-a-time. ഀ |
References ഀ |
| ഀ
[1] T. K. Sato, M. Asai, A. Borschevsky, T. Stora, N. Sato, Y. Kaneya K. Tsukada, Ch. E. Düllmann, K. Eberhardt, E. Eliav, S. Ichikawa, U. Kaldor, J. V. Kratz, S. Miyashita, Y. Nagame, K. Ooe, A. Osa, D. Renisch, J. Runke, M. Schädel, P. Thörle-Pospiech, A. Toyoshima, N. Trautmann, “Measurement of the first ionization potential of lawrencium (element 103)”, Nature XXX, XXX-XXX (2015). ഀ ഀ[2] M. Schädel, Phil. Trans. R. Soc. A 373, 20140191 (2014), and M. Schädel, D. Shaughnessy (Edts.), The Chemistry of Superheavy Elements, 2nd Edition, Springer, Heidelberg (2014). ഀ ഀ[3] T. K. Sato et al., Rev. Sci. Instrum. 84, 023304 (2013). ഀ ഀ[4] T. K. Sato et al., J. Radioanal. Nucl. Chem. 303, 1253 (2015). ഀ ഀ[5] N. Sato et al., Radiochim. Acta 102, 211 (2014). ഀ |
ഀ