The presence of the actinides
Th, U, Np, Pu, and Am in transuranic (TRU) and mixed wastes is a major
concern because of their potential for migration from the waste repositories
and long-term contamination of the environment. The actinides in TRU and
mixed wastes may be present in various forms, such as elemental, oxide,
coprecipitates, inorganic, and organic complexes, and as naturally occurring
minerals depending on the process and waste stream. The actinides exist
in various oxidation states: Th (III, IV); U (III, IV, V, VI); Np (III,
IV, V, VI, VII); Pu (III, IV, V, VI, VII); and Am (III, IV, V, VI, VII).
In addition to the radionuclides the TRU waste consists a variety of
organic materials (cellulose, plastic, rubber, chelating agents) and inorganic
compounds (nitrate and sulfate).
Significant microbial activity is expected in the waste
because of the presence of organic compounds and nitrate, which serve
as carbon and nitrogen sources and in the absence of oxygen the microbes
use nitrate and sulfate as alternate electron acceptors.
Biodegradation of the TRU waste can result in gas generation and pressurization
of containment areas, and waste volume reduction and subsidence in the
repository. Although the physical, chemical, and geochemical processes
affecting dissolution, precipitation, and mobilization of actinides have
been investigated, we have only limited information on the effects of
microbial processes.
Microorganisms have been detected in TRU wastes, Pu-contaminated soils,
low-level radioactive wastes, backfill materials, natural analog sites,
and waste-repository sites slated for high-level wastes. Microbial activity
could affect the chemical nature of the actinides by altering the speciation,
solubility and sorption properties and thus could increase or decrease
the concentrations of actinides in solution. Actinides may be present
initially as soluble or insoluble forms and, after disposal, may be converted
from one to the other by microorganisms. Under appropriate conditions,
actinides can be solubilized or precipitated by direct (enzymatic) or
indirect (nonenzymatic) actions of microorganisms.
These include (i) oxidation-reduction reactions, (ii) changes in pH
and Eh, (iii) chelation, or the production of specific sequestering agents,
(iv) biosorption and bioaccumulation by biomass and biopolymers, (v) bioprecipitation
reactions leading to the formation of stable minerals, and (vi) biotransformation
of actinides complexed with organic and inorganic ligands. Free-living
bacteria suspended in the groundwater fall within the colloidal size range
and may have strong radionuclide sorbing capacity, giving them the potential
to transport radionuclides in the subsurface. Microbial activities are
influenced by electron donors and acceptors and the extent of dissolution
and precipitation could be significant, particularly under anaerobic conditions.
In anaerobic environments, actinides can be reduced enzymatically from
a higher oxidation state to a lower one, which affects their solubility
and bioavailability. For example, reduction of U6+ to U4+
decreases its solubility. Key microbial processes involved in the mobilization
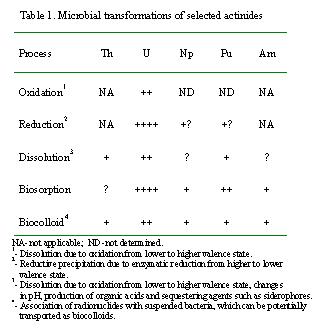
or immobilization of selected actinides of interest is summarized in Table
1. Among the actinides, biotransformation of uranium has been extensively
studied, whereas we have only limited understanding of the microbial transformations
of other actinides such as Th, Np, Pu, and Am present in TRU and mixed
wastes [1, 2].
Chelating agents are present in TRU and mixed wastes
because they are widely used for decontaminating nuclear reactors and
equipment, in cleanup operations, and in separating radionuclides.
Many organic compounds form stable complexes with actinides, and increase
their solubilization and leaching. Plutonium forms very strong complexes
with a variety of organic ligands. Naturally occurring organic complexing
agents, such as humic and fulvic acids, and likewise microbially produced
complexing agents, such as citrate, and siderophores, as well as synthetic
chelating agents and the products or intermediates from waste degradation
may be an important source of agents affecting the solubility and mobility
of actinides.
Biotransformation of actinide-organic complexes should result in the
degradation of the organic ligand and precipitation of the actinide [3].
Remediation of Radionuclide Contaminated Soils
and Wastes, and Materials.
Fundamental understanding of the mechanisms of microbiological transformations
of various chemical forms of uranium present in wastes and contaminated
soils and water has led to the development of novel bioremediaition processes.
One process uses anaerobic bacteria to stabilize the radionuclides and
toxic metals from the waste, with a concurrent reduction in volume due
to the dissolution and removal of nontoxic elements from the waste matrix.
In an another process, uranium and other toxic metals are removed from
contaminated soils and wastes by extracting with the chelating agent citric
acid. Uranium is recovered from the citric acid extract after biodegradation/photodegradation
in a concentrated form as UO3×2H2O for recycling or appropriate disposal
[4, 5].
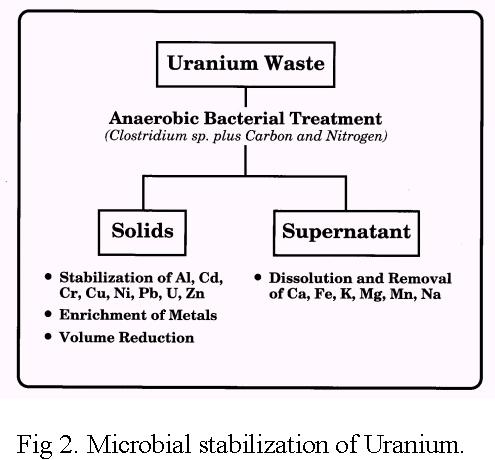
Stabilization of Uranium by Reductive Precipitation
by Anaerobic Bacteria.
Immobilization of uranium is brought about by bioreduction and bioprecipitation
reactions. Uranium is reduced by a wide variety of facultative and strict
anaerobic bacteria under anoxic conditions in the presence of suitable
electron donors.
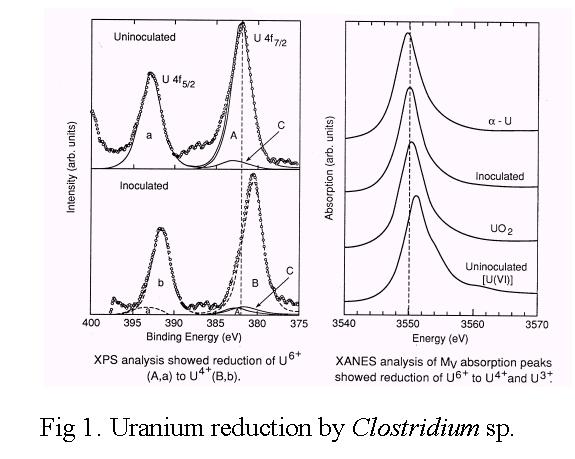
![]()
Speciation of uranium in microbial cultures by x-ray
absorption near edge spectroscopy (XANES) and X-ray photoelectron spectroscopy
(XPS) showed that soluble U(VI) was reduced to insoluble U(IV) by the
anaerobic bacterium, Clostridium sp [Fig 1]. Treatment of uranium- and
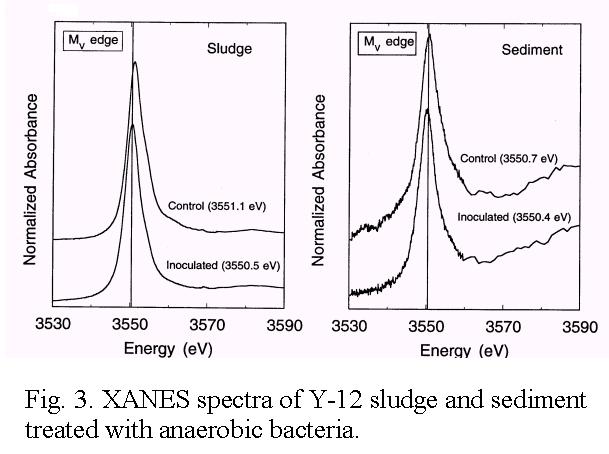
toxic-metal contaminated sediment and sludge with the anaerobic bacterium
Clostridium sp. removed a large fraction of soluble non-toxic metals such
as Ca, K, Mg, Mn2+, Na, and Fe2+, enriched and stabilized
Cd, Cr, Cu, Ni, Pb, U and Zn, and reduced the overall volume and mass
[Figs. 2 and 3]. In this novel approach to treating wastes, the unique
metabolic capabilities of the dual-action anaerobic bacteria were exploited
to solubilize and/or precipitate radionuclides and toxic metals directly
by enzymatic action and indirectly by the production of organic acid metabolites.
The non-hazardous materials in the solid phase
were solubilized and removed from the waste, thereby reducing its volume.
The remobilized radionuclides and toxic metals are stabilized by precipitation
reactions and redistributed with stable mineral phases of the waste.
Consequently, the potential exists for he use of anaerobic bacteria to concentrate, contain and stabilize U in contaminated groundwaters and in waste with concurrent reduction in waste volume. Reactive barrier technology is based on the activities of these anaerobic bacteria. However, the long-term stability of bacterially immobilized U in the natural environment is poorly understood.
Removal and Recovery of uranium from contaminated
soils and wastes.
Citric acid, a naturally occurring compound, is a multidentate ligand,
which forms stable complexes with various metal ions.
It forms different types of complexes with transition metals and actinides
including formation of a bidentate, tridentate, binuclear, or polynuclear
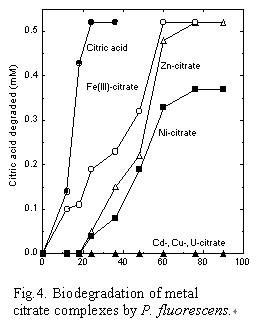
complex species. Biodegradation of metal citrate complexes is dependent
upon the type of complex formed between the metal and citric acid; bidentate
complexes are readily biodegraded whereas the tridentate complexes are
recalcitrant [3]. Pseudomonas fluorescens metabolized the bidentate complexes
whereas complexes involving the hydroxyl group of citric acid, and the
binuclear U-citrate complex are not [Fig. 4].
The presence of the free hydroxyl group of citric acid is the key determinant
in effecting biodegradation of the metal complex. The lack of degradation
was not due to their toxicity but was limited by the transport and/or
metabolism of the complex by the bacteria. No relationship was observed
between biodegradability and stability of the complexes.
For decontamination, uranium must be removed and recovered from the contaminated
site, so that the site is restored. Various soil washing techniques have
been developed including physical methods, such as wet-screening, attrition
scrubbing, or chemical methods consisting of treating with organic and
inorganic acids, salts, bases, and chelating agents. For example, nitric
acid, hydrochloric acid, phosphoric acid, sulfuric acid, sodium carbonate,
ammonium carbonate, sodium hydroxide, oxalic acid, citric acid, EDTA,
and DTPA have been used to extract radionuclide and toxic metals. Many
of the inorganic chemicals used are corrosive, which irreparably damages
the soil. Furthermore, all chemical extraction methods generate secondary
waste streams which create further problems of hazardous waste disposal.
Among the several organic complexing agents used in extracting metals,
citric acid appears to be the most preferred because it is a naturally
occurring organic complexing agent. It is environmentally friendly, exhibits
relatively consistent removal efficiency, and is cost-effective. Citric
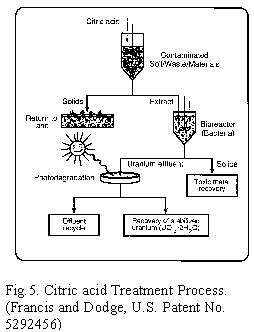
acid extract is subjected to biodegradation, followed by photodegradation
[Fig.5]. Several metal citrate complexes are readily biodegraded, and
the metals are recovered in a concentrated form, along with the bacterial
biomass. Uranium forms a binuclear complex with citric acid and is recalcitrant.
The supernatant containing this complex is separated, and exposed to
light; it rapidly degrades with the precipitation of uranium. Uranium
is recovered as UO3×2H2O in a concentrated form for recycling, or for
disposal [Fig. 5]. This treatment process, unlike others, does not generate
additional hazardous wastes for disposal and causes little damage to the
soil which is then be returned to normal use.
This process has significant potential for commercialization because
(i) it can be applied to a variety of materials and waste forms; (ii)
mixed waste is separated into radioactive and hazardous waste; (iii) uranium
is separated from the toxic metals and recovered for recycling or disposal;
(iv) it does not generate secondary waste streams; (v) it causes little
damage to soil; and (vi) environmentally and economically important metals
are removed in a concentrated form. The use of combined chemical, photochemical,
and microbiological treatments of contaminated materials will be more
efficient than present methods and result in considerable savings in clean
up and disposal costs.
Summary
Microorganisms can alter the stability and mobility of actinides in radioactive
wastes and in the natural environment. Such microbial transformations
of uranium have been extensively studied. The direct implication of microorganisms
in precipitating actinides is important because of the potential application
in bioremediating contaminated sites, in pre-treating radioactive wastes,
and in processes critical to nuclear-waste repositories. Although a wide
variety of microorganisms are present in radioactive wastes and natural
radioactive mineral deposits, the extent to which they regulate the mobility
of the actinides is not fully understood. Furthermore, the effects of
microbial activities on TRU and mixed waste and their potential for treatment
of certain waste forms to stabilize the actinides and reduce the volume
of the waste have not been fully exploited.
Fundamental understanding of the mechanisms of microbial transformations
of different chemical forms of actinides under various environmental and
microbial process conditions such as aerobic, anaerobic (denitrifying,
fermentative, and sulfate reducing) and repository relevant conditions
will be useful in predicting the long-term performance of waste repositories
and in developing novel strategies for waste management and remediation
of contaminated sites.
Acknowledgment. This research was sponsored by the Environmental Remediation Sciences Division, Office of Biological and Environmental Research, Office of Science, U.S. Department of Energy under contract No. DE-ACO2-76CH00016.
References
1. Francis, A.J. 2001. Microbial transformations of plutonium and implications
for its mobility. In“Plutonium in the Environment” A. Kudo, (Ed) Elsevier
Science Ltd., Co., UK. Pp 201-219.
2. Francis, A.J., C.J. Dodge, F. Lu, G. Halada, and C.R. Clayton. 1994.
XPS and XANES studies of uranium reduction by Clostridium sp. Environ.
Sci. Technol. 28:636-639.
3. Francis, A.J., C.J. Dodge, J.B. Gillow. 1992. Biodegradation of metal
citrate complexes and its
implications for toxic metal mobility. Nature, 356:140-142.
4. Francis, A.J. and C.J. Dodge 1998. Remediation of soils and wastes
contaminated with uranium and toxic metals. Environ. Sci. Technol.32:
3993-3998.
5. Francis, A.J. 1999. Bioremediation of radionuclide and toxic metal
contaminated soils and wastes. In
Bioremediation of Contaminated Soils, pp 239-271. Agronomy Monograph No.
37. ASA, CSSA, SSSA, Madison, WI.
![]()